The general idea of electroplating is to use electricity to coat a relatively mundane or dull metal, such as copper, with a thin later of another more precious metal, such as gold or silver.
What is electroplating?
It’s passing an electric current through a solution called an electrolyte. This works by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit into a power supply, in which most cases a battery is mostly used.
When the electricity flows through the circuit made, the electrolytes splits up and some of the metal atoms it contains are transferred into a thin layer on top of one of the electrodes, making it electroplated.
What does it really do?
Electroplating is used to make things resistant to rust. Another one of its function is to produce a variety of useful alloys like brass and bronze, and even make plastic look like metal. It uses anodic and cathodic processes to bond compatible metals to the substrate like a zinc over a raw steel part. The topcoats such as zinc and nickel prevent it from rusting or at least serve as a coating that corrodes at a much slower rate, in other words it prevents rusting to lengthen the serve time of the object.
For some applications, electroplating can serve as decorative function too. It’s cheaper to have gold or silver plated jewelry than solid items made from these heavy, expensive and precious metals.
Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming.
Electroplating vs. Electrolysis
They’re both examples of electrochemistry. Although they’re similar to one another, as the idea of electrolysis is using electricity to light up a chemical solution, electroplating is the reverse process of electrolysis by which batteries produce electric currents.
How does “electroplating” work?
First and foremost, you have to choose compatible electrodes and electrolyte by figuring out the chemical reaction(s) you want to happen when the electric current is switched on.
Second, cleanliness must be assured. If not, when metal atom from the electrolyte are deposited onto it, they won’t form a good bond and they may simply rub off again. You can make sure for it to be clean by by dipping the electrode into a strong acid or alkaline solution.
Cleanliness assures the atoms from the plating metal bond effectively joins onto the outside edges of its crystalline structure.
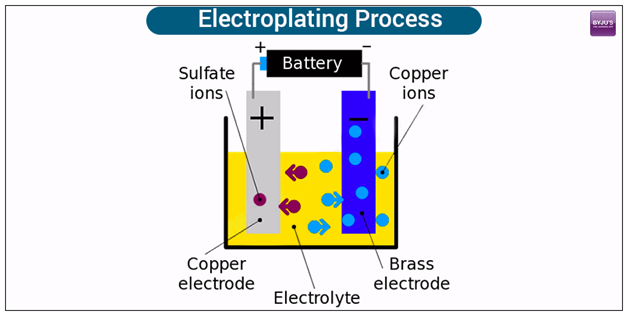
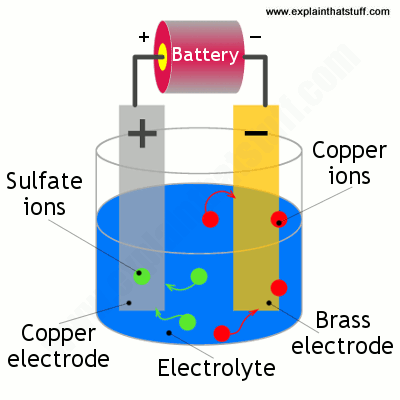
Now we need to prepare two electrodes of conducting material, an electrolyte, and an electric supply. In a general experiment, one of the electrodes is made of the metal we’re trying to plate and the electrolyte is a solution of a salt of the same metal.
– Example –
An example would be trying to plate brass. We would need a copper electrode, a brass electrode, and a solution of a copper-based compound such as copper sulfate solution.
Start off by dipping the two electrodes into the solution and connect them into a circuit so the copper becomes anodic and the brass becomes cathodic. When the power is on, the copper sulfate solution splits into ions, and these ions are attracted to the negatively charged brass electrode and slowly deposit on it. Hence, producing a copper plate. As for the sulfate ions, they will release electrons moving through the battery toward the negative brass electrode.
How long it takes for electroplated atoms to build up on the surface of the negative electrode depends on the strength of the electric current used and the concentration of the electrolyte. Increase in either of these increases the speed of the movement of ions and electrons through the circuit and eventually speed up the plating process. As long as movements go on, current keeps flowing and the plating process goes on too.
Electroplating Plastics
Plastics are non-conductors or insulators. Although they can be plated, they need a “boost”.
To begin with, they need to be EXTRA clean from dust, dirt, grease and surface marks. In order to allows coating to take place and for it to stick on the surface, they also need to be etched with acid and treated with catalyst. They are commonly dipped in copper or nickel to give it a very thin coating of electrically conducting metal before they’re electroplated just like a metal.
Many different plastics can be plated this way, including ABS, nylon and poly-carbonate. They are generally used as parts of cars, plumbing, household, and electrical fittings.
Some of its benefits compared to actual metals are that they are lighter, cheaper, rustproof, and don’t require polishing after plating.